Clinical Trials and Real-World Data
The Clinical Trials Conducted Prove the Effectiveness, Safety, and Ease-of-Use of the SyqeAir Inhaler.
Main Conclusions from The Studies:
- Low and metered doses – The cannabis Inhaler provides metered and low doses of Δ9-THC to reduce chronic neuropathic pain with no serious adverse events and enables the administration of personalized treatment with medical cannabis1
- Easy to use – Using the Inhaler is convenient, easy, and simple, according to hospitalized patients2
- Fast and consistent reduction in pain intensity, with minimal adverse effects – The SyqeAir Inhaler provides a metered dose of THC for a fast and significant reduction in pain indices, a uniform and consistent effect with almost no reports of psychoactive adverse effects (or none at all)1,3
- Improvement in quality of life – Long-term treatment with the SyqeAir Inhaler provides a general reduction in the intensity of pain and an improvement in the quality of life while maintaining a much better safety profile compared to the older methods of consumption (oil and smoking/vaping)4-5
- Stable and consistent effect – Adjusting the appropriate dosage regimen for the patient by a Syqe® nurse has a positive impact on producing a stable and consistent treatment over time4-5
- Safe to use – The reduction in pain intensity, as reported by SyqeAir patients, is equivalent to treatment with 20-40 grams of cannabis per month4-5, while the daily dose of THC inhaled by the patients is 79 times lower (!) compared to THC-rich cannabis products6
- Improvement in sleep quality – The sleep latency has shortened, and the sleep duration has prolonged, on average5
- Almog S, et al. (2020) European Journal of Pain, 24(8), 1505-1516.
- Vulfsons, S. et al. (2020). Palliative & Supportive Care, 18(1), 12-17.
- Eisenberg, E, et al. (2014). J Pain Palliat Care Pharmacother, 28(3), 216-225.
- Aviram J, et al. (2022). PAIN Reports. 7(3). e1011.
- Aviram, J, et al., (2023). Pharmaceuticals, 16(1426), 1-15.
- Aviram J, et al. (2021). Pharmacological Research. 169(105651). 1-10.
The Following Data Demonstrate the Principles of the Trials Conducted by the Company
The Pharmacokinetics, Efficacy, and Safety of a Novel Selective-Dose Cannabis Inhaler in Patients With Chronic Pain: A Randomized, Double-Blinded, Placebo-Controlled Trial
Background
Cannabis treatment with metered dosing remains a major challenge, leading to physicians’ reluctance to prescribe medical cannabis.
Objective
To test the pharmacokinetics, analgesic effect, cognitive performance, and safety effects of an innovative medical device that enables the delivery of inhaled therapeutic doses of Δ9-Tetrahydrocannabinol (THC) in patients with chronic pain.
Methods
In a randomized, three-arms, double-blinded, placebo-controlled, cross-over trial, 27 patients received a single inhalation of Δ9-THC: 0.5mg, 1mg, or a placebo.
Δ9-THC plasma levels were measured at baseline and up to 150-min post-inhalation. Pain intensity and safety parameters were recorded on a 10-cm visual analogue scale (VAS) at pre-defined time points. The cognitive performance was evaluated using the selective sub-tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Results
Following inhalation of 0.5 mg or 1mg, Δ9-THC plasma Cmax ± SD were 14.3 ± 7.7 and 33.8 ± 25.7 ng/ml. Tmax ± SD were 3.7 ± 1.4 and 4.4 ± 2.1 min, and AUC0 → infinity±SD were 300 ± 144 and 769 ± 331 ng*min/ml, respectively. Both doses, but not the placebo, demonstrated a significant reduction in pain intensity compared with baseline and remained stable for 150-min. The 1-mg dose showed a significant pain decrease compared to the placebo. Adverse events were mostly mild and resolved spontaneously. There was no evidence of consistent impairments in cognitive performance.
Conclusion
This feasibility trial demonstrated that a metered-dose cannabis inhaler delivered low and metered THC doses, produced a dose-dependent and safe analgesic effect in patients with neuropathic pain/ complex-regional pain syndrome (CRPS). Thus, it enables individualization of medical cannabis regimens that can be evaluated pharmacokinetically and pharmacodynamically by accepted pharmaceutical models.
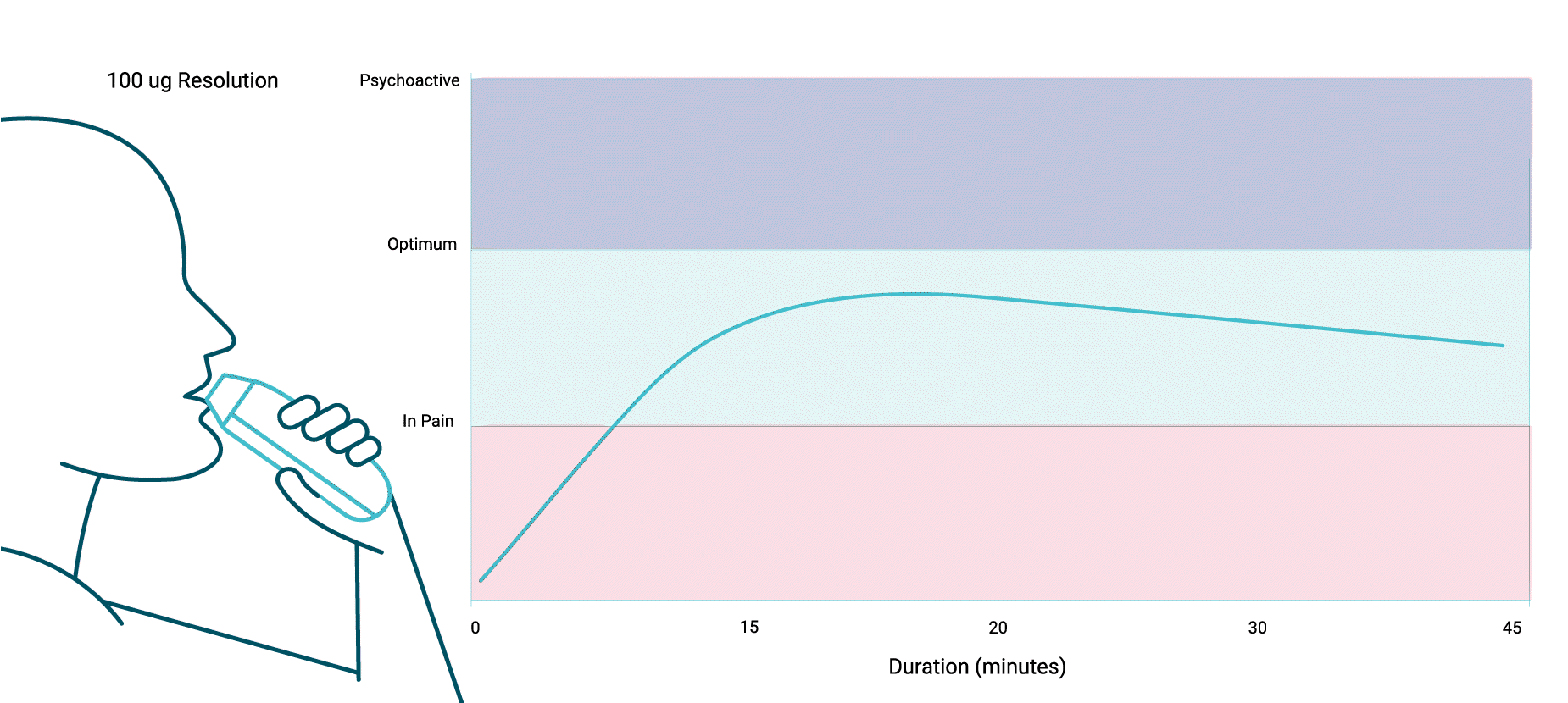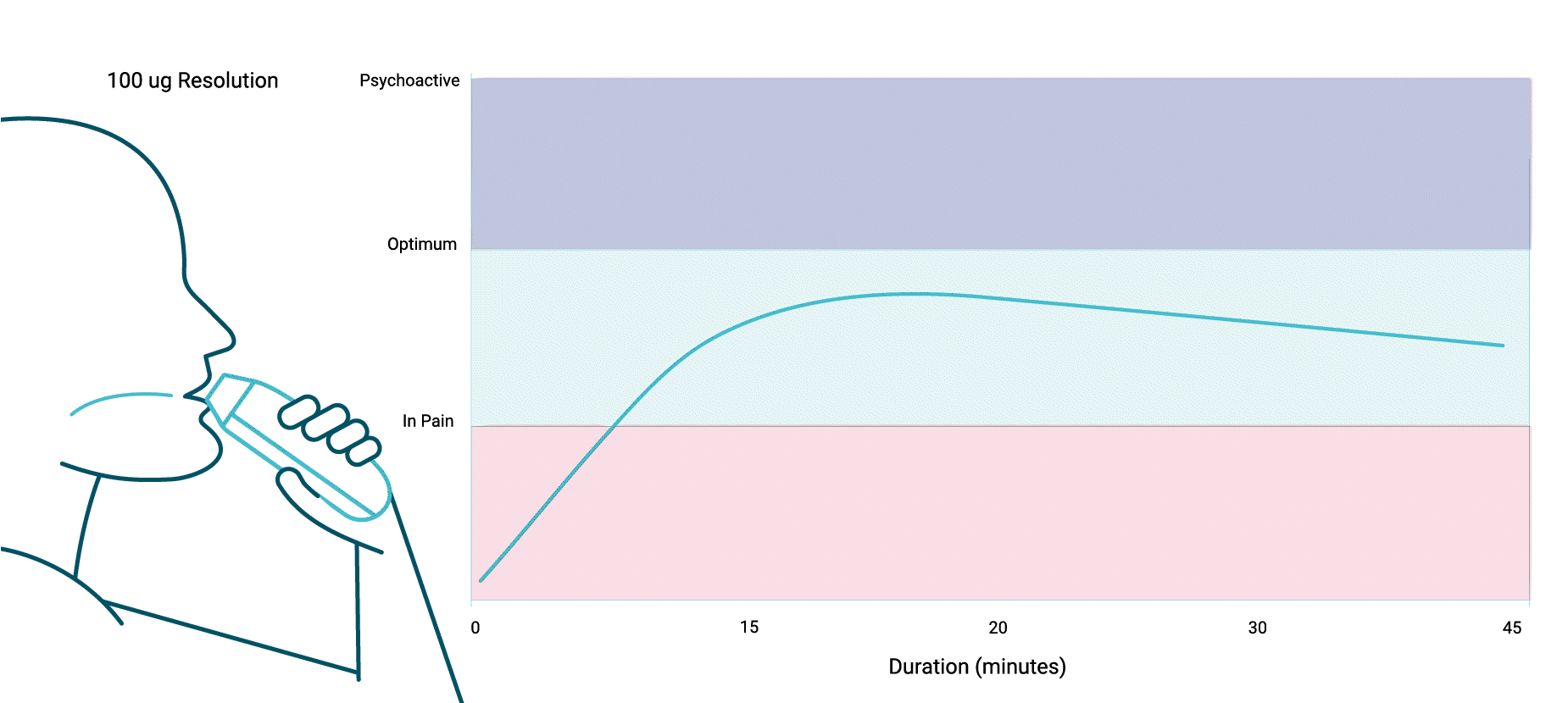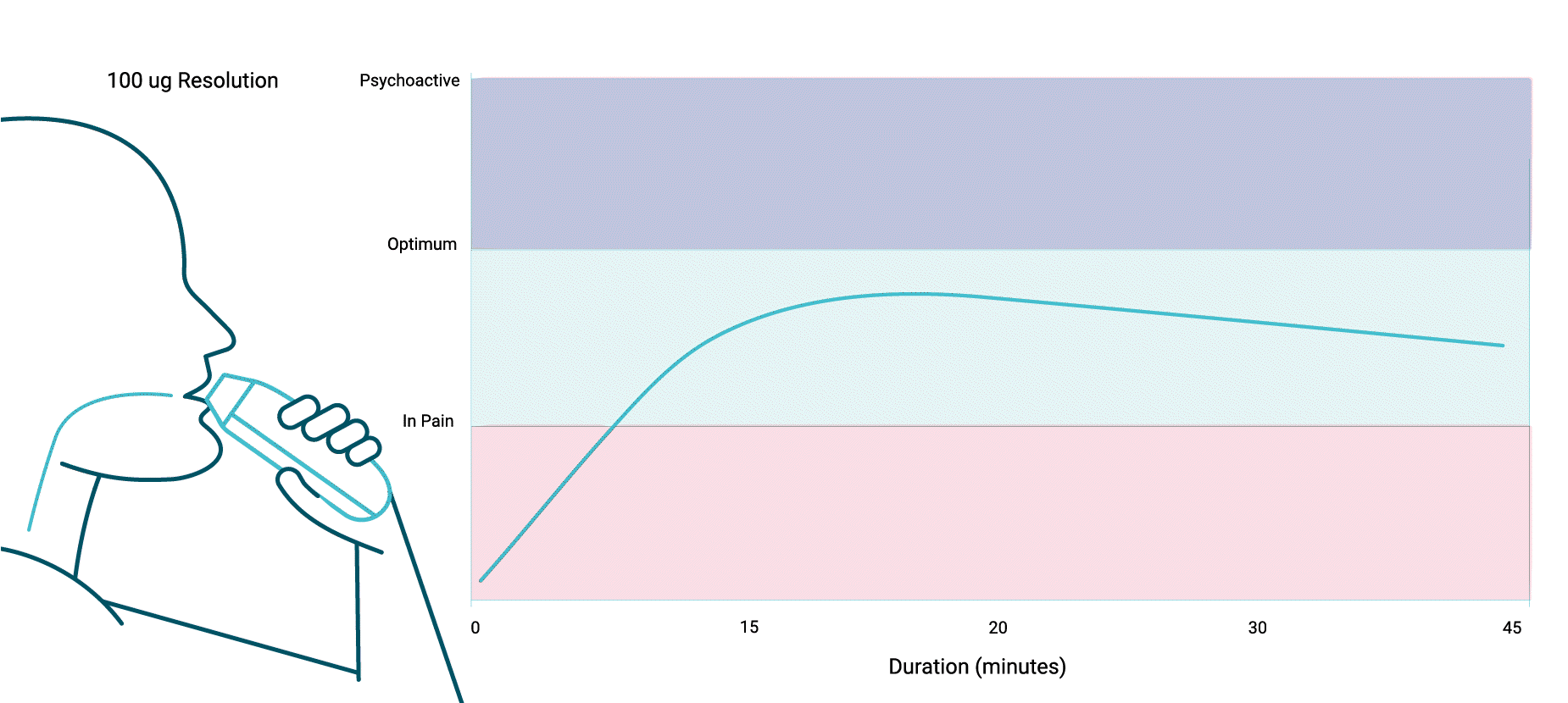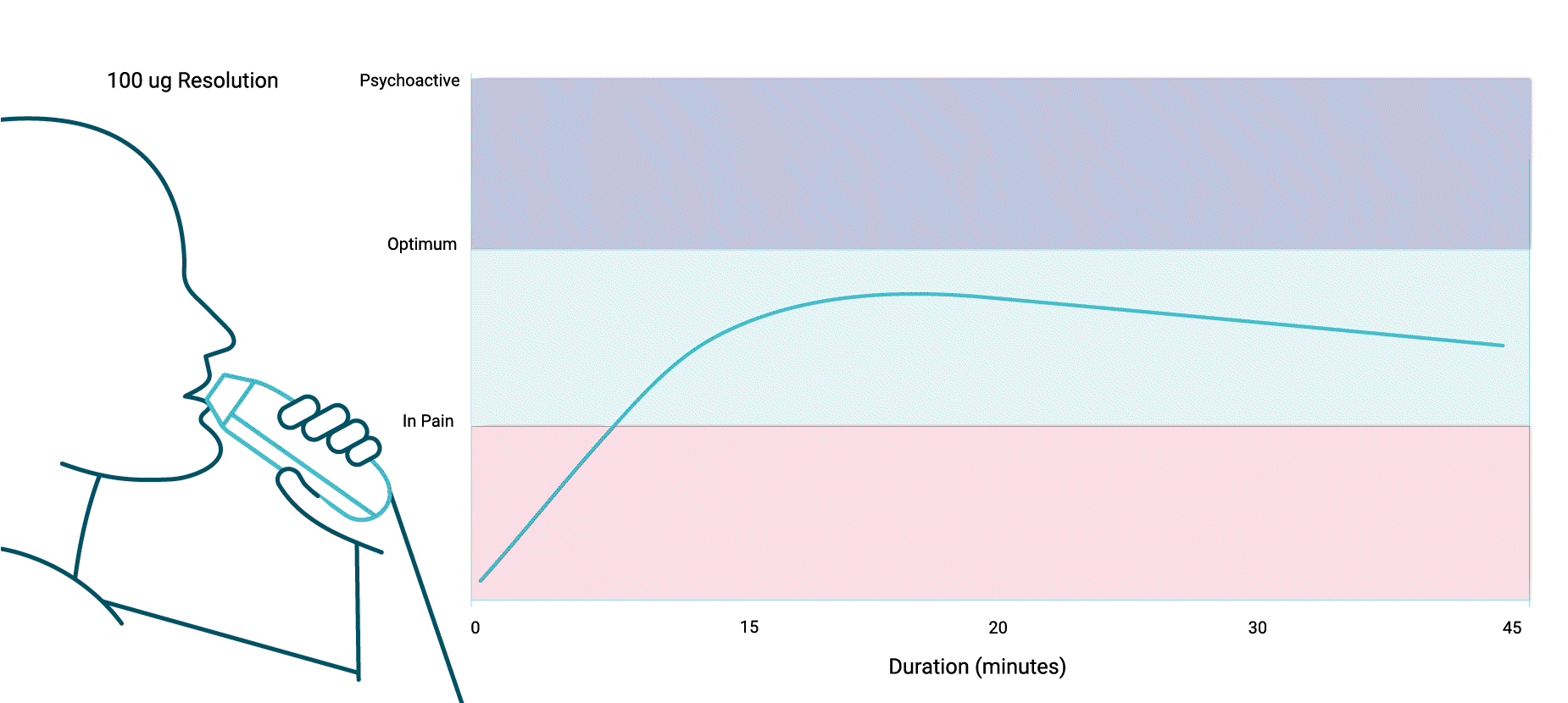
Cannabis Treatment in Hospitalized Patients Using the SYQE® Inhaler: Results of a Pilot Open-Label Study
A questionnaire study was conducted in Rambam Medical Center in a cohort of hospitalized patients that were using medical cannabis under license as a part of their ongoing medical treatment that were not able to use cannabis while being admitted to the hospital and therefore were prescribed with Syqe®’s inhaler during their hospitalization.
Objective
Evaluation of the usability, feasibility of use, and satisfaction of the inhaler among hospitalized patients and the medical staff attending to them. The participants used medical cannabis utilizing the Syqe®-EXO Inhaler as a part of their ongoing medical treatment. The treatment was prescribed to the patients by doctor treating their ward.
Method
The participants used the inhaler from the moment of their admission to the hospital and until their release. The use varied between 1-7 days. Prescribed treatment included 4 daily inhalations and up to an additional 3 SOS inhalations (distress code for more doses of cannabis). In each inhalation, the patient received a 500 μg dose of THC extracted from the cannabis flos.
A patient satisfaction questionnaire and a usability questionnaire were filled in following the last use. The medical staff which was involved in providing the inhalers to the patients, also filled in a questionnaire on the inhaler’s ease-of-use and satisfaction.
Results
Patients were easily trained on how to use the inhaler and no malfunctions were registered.
Conclusions
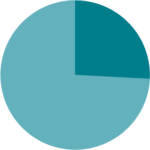
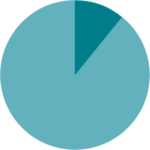
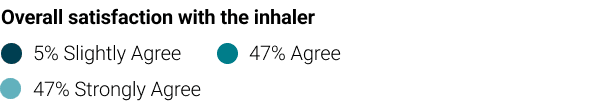
High levels of satisfaction from the inhaler were yielded, both from patients and medical staff, and they found it to be convenient and easy to use during hospitalization.



The Pharmacokinetics, Efficacy, Safety, and Ease of Use of a Novel Portable Metered-Dose Cannabis Inhaler in Patients with Chronic Neuropathic Pain: A Phase 1a Study
Objective
Exploring the pharmacokinetics (the course of drug in the body), safety, tolerability, efficacy, and ease of use of a portable thermal-metered-dose inhaler (tMDI) delivering metered doses of THC on pain intensity.
The trial was conducted among a group of patients suffering from chronic and neuropathic pain and on a stable analgesic regimen including medicinal cannabis, licensed by the Ministry of Health. The trial also included an estimation of pain and different safety parameters, in addition to measuring the concentration of THC in the blood. The participants abstained using cannabis in the 12 hours prior to the start of the trial.
Methods
All participants inhaled a single dose, after abstaining from cannabis for 12 hours. Blood samples for Δ9-THC and 11-hydroxy-Δ9-THC were taken at baseline and up to 120 minutes. Pain intensity (0–10 VAS), adverse events, and satisfaction score were monitored following the inhalation.
Measured: concentration of active substances (THC) in the blood, change in the level of pain, changes in the indices: blood pressure, pulse and more.
Results
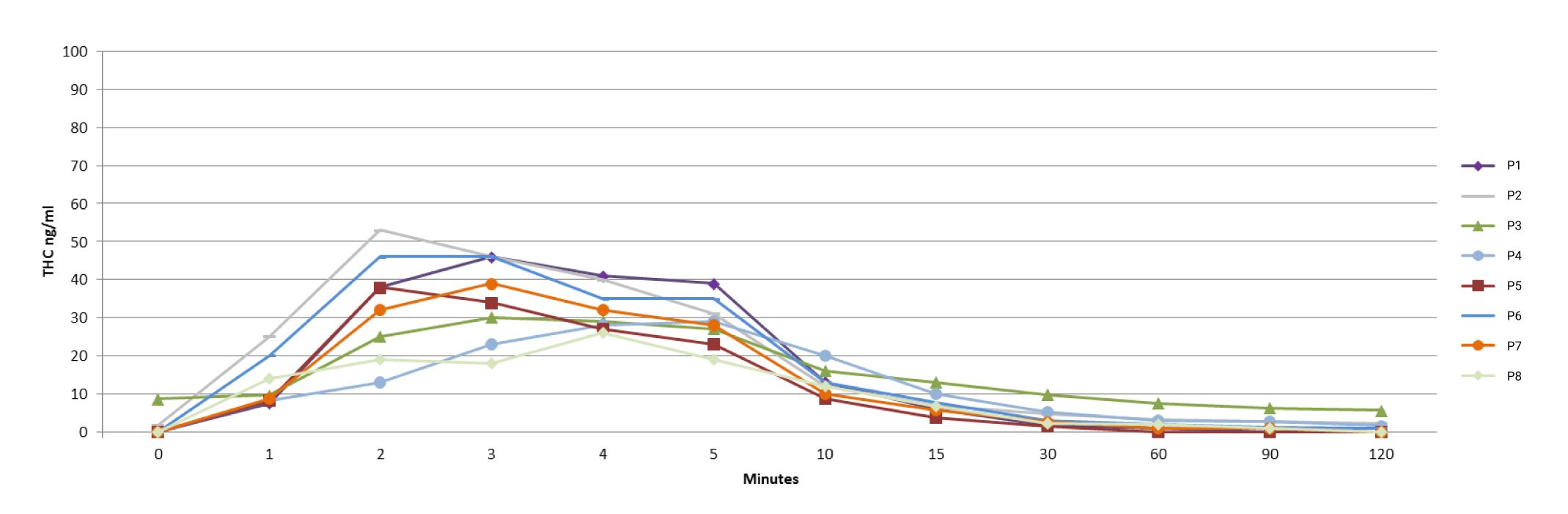
Compared to all methods of cannabis administration by inhalation examined in other studies around the world, Syqe®’s inhaler was found to provide the most uniform results in the THC plasma Cmax among patients.
A significant 45% reduction in pain intensity was noted 20 minutes post inhalation.
Tolerable, lightheadedness, lasting 15–30 minutes and requiring no intervention, was the only reported adverse event. In addition, compared to the basic level of mean systolic blood pressure, a significant decrease was measured, 30 minutes after inhalation.
On average, patients expressed high satisfaction with using the inhaler, compared to their current cannabis method of administration (smoking).
Conclusions
The results prove that Syqe®’s inhaler provides a metered dose of THC demonstrated in a uniform pharmacokinetic profile, and it allows for significant reduction in pain levels. Other studies examining THC concentration in the blood after smoking cannabis have found high variation among patients. These concentrations were accepted despite the effort to control the inhalation variables, including depth and length of the inhalation, duration of holding the breath, duration of exhalation, and the time between one inhalation and another.
In addition, these studies have shown relatively high concentrations of THC in the blood, effecting on the amount of adverse events in general, and psychoactive adverse events in particular. In contrast, treatment utilizing Syqe®’s inhaler demonstrated rapid and accurate results with no report of psychoactive adverse events.

Long-Term Effectiveness and Safety of Medical Cannabis Administered Through the Metered-Dose Syqe® Inhaler
Introduction
The first three clinical trials conducted by Syqe® dealt with medical cannabis treatment using the Metered-Dose SyqeAir Inhaler. Their results demonstrated the short-term effectiveness and safety of the treatment at very low and metered doses of medical cannabis. This fourth study is not a clinical trial but a real-world evidence data collection study with the participation of SyqeAir’s patients.
Objective
Understand and outline the therapeutic effect (effectiveness and safety) of medical cannabis administrated through the SyqeAir Inhaler – in a retrospective analysis of “real-life” data collected in real-time during the long-term treatment of patients who willingly agreed to participate in the study.
Methods
Syqe®’s Nursing team, which personally guides and supports each patient, collected data on the intensity of pain reported by the patients from the start of the treatment to 120 days after treatment initiation. In addition, active and passive follow-ups were conducted on adverse effects over a period of 15 months, and a record has been made of the change in the quality of life during treatment using the SyqeAir Inhaler.
Tetrahydrocannabinol (THC) served as a dosage marker for the rest of the ingredients of the full-spectrum cannabis inflorescence. SyqeAir Cartridges contain T20C4 medical cannabis from adherent, uniform, and genetically stable inflorescences grown by Bedrocan®. All patients consumed their personalized dosage according to their dosage regimen adjusted to them by a Syqe® nurse.
Results
The study collected data from 143 patients with an average age of 62 years; 54% were men, most (72%) were treated with the Inhaler for chronic neuropathic pain. The average daily dose consumed was 1,500 mcg of THC (along with the rest of the ingredients of a full-spectrum inflorescence).
- Compared to the pain levels before the start of the treatment, the patients reported a significant reduction in pain intensity of 22.8% on average.
- Patients with severe pain (8 or more on a 0-10 scale) reported an average reduction in pain intensity of 28.4% (a decrease of 2.41 points on a 0-10 scale).
- 92% of the patients reported an improvement in their quality of life.
- Adverse effects were mostly reported during the dosage adjustment period (by 34% of the patients), while during the three months that followed, only 4% of patients reported adverse effects. Over the 15-month follow-up period, there were points in which no adverse effects were reported at all.
- Only 7% of patients reported psychoactive adverse effects associated with medical cannabis treatment, which included anxiety and restlessness only.
Conclusions
The long-term treatment using the SyqeAir Inhaler provides a general reduction in the intensity of pain and an improvement in the quality of life while maintaining a much better safety profile compared to the conventional routes of administration (oil, smoking, and vaping).
- There is a stable and consistent effect over time, following the dosage adjustment period with the help of a nurse.
- The reduction in pain intensity reported by patients who use the SyqeAir Inhaler is equivalent to treatment with 20-40 grams of cannabis per month – The daily dose of THC inhaled by the patients was 79X times lower (!) compared to THC-rich cannabis products. This number also leads to the conclusion that, apparently, medical cannabis has an “effect limit” of 2-3 points of reduction in pain intensity, which cannot be crossed even with high doses of cannabis consumed by smoking, vaporizing, or digestive oil.
- Patients who want to maintain the optimal balance between reducing the intensity of pain and minimal adverse effects (if at all) can do so using SyqeAir Medical Cannabis Inhaler.
Research Innovation
For the first time, we are conducting research with medical cannabis, relying on the following important variables:
- Optimal accuracy: We know the exact dose of THC (in mcg) that the patients regularly consume.
- Authenticity: This is a long-term, “real-world” treatment conducted in routine conditions. This is compared to the “sterile” laboratory conditions that characterize clinical trials. All patients inhaled a dose adjusted to them by a nurse and prescribed to them by their attending physician according to their specific medical condition.
- Adherence and consistency: All participants inhaled medical cannabis of the same specific strain, which is adherent and has a consistent effect. They all consumed the cannabis using the Metered-Dose SyqeAir Inhaler; This is in comparison to studies not conducted by Syqe®, where the participants take different strains in imprecise and consistent doses without enabling the researchers to discern the real concentrations of the active ingredients consumed by the patients; And also the routes of administration used by the patients in the other studies are varied (smoking with/without tobacco, different types of vaporizers, etc.)

THC Degradation Does Not Impair the Accuracy of THC Doses Aerosolized by the Metered-Dose SyqeAir Inhaler: A 24-Month Stability Trial
Background
Although the worldwide use of medical cannabis (MC) is on the rise, there is insufficient data regarding the long-term stability of phytocannabinoids in the plant material under different storage conditions. Specifically, there is insufficient data on the effect of storage conditions on the availability of (-)-∆9-trans-tetrahydrocannabinol (THC) in vaporized cannabis. The SyqeAir Inhaler delivers metered doses of phytocannabinoids by inhalation and utilizes accurate quantities of ground cannabis inflorescence packaged in tamper-proof Cartridges. We aimed to assess the stability of phytocannabinoids in ground cannabis before and after packaging in the SyqeAir Cartridges, as well as the reproducibility of THC delivery in the aerosolized dose.
Methods
Ground medical cannabis inflorescence was stored under different temperature and humidity conditions before or after being packaged in the SyqeAir Cartridges. Concentrations of the major phytocannabinoids therein were analyzed at different time points using ultra-high performance liquid chromatography (U-HPLC). THC doses aerosolized via the SyqeAir Inhaler were evaluated using Cartridges stored for up to 2 years at 25°C. Every VaporChip contains 13.5±0.9 mg of ground medical cannabis powder.
Results
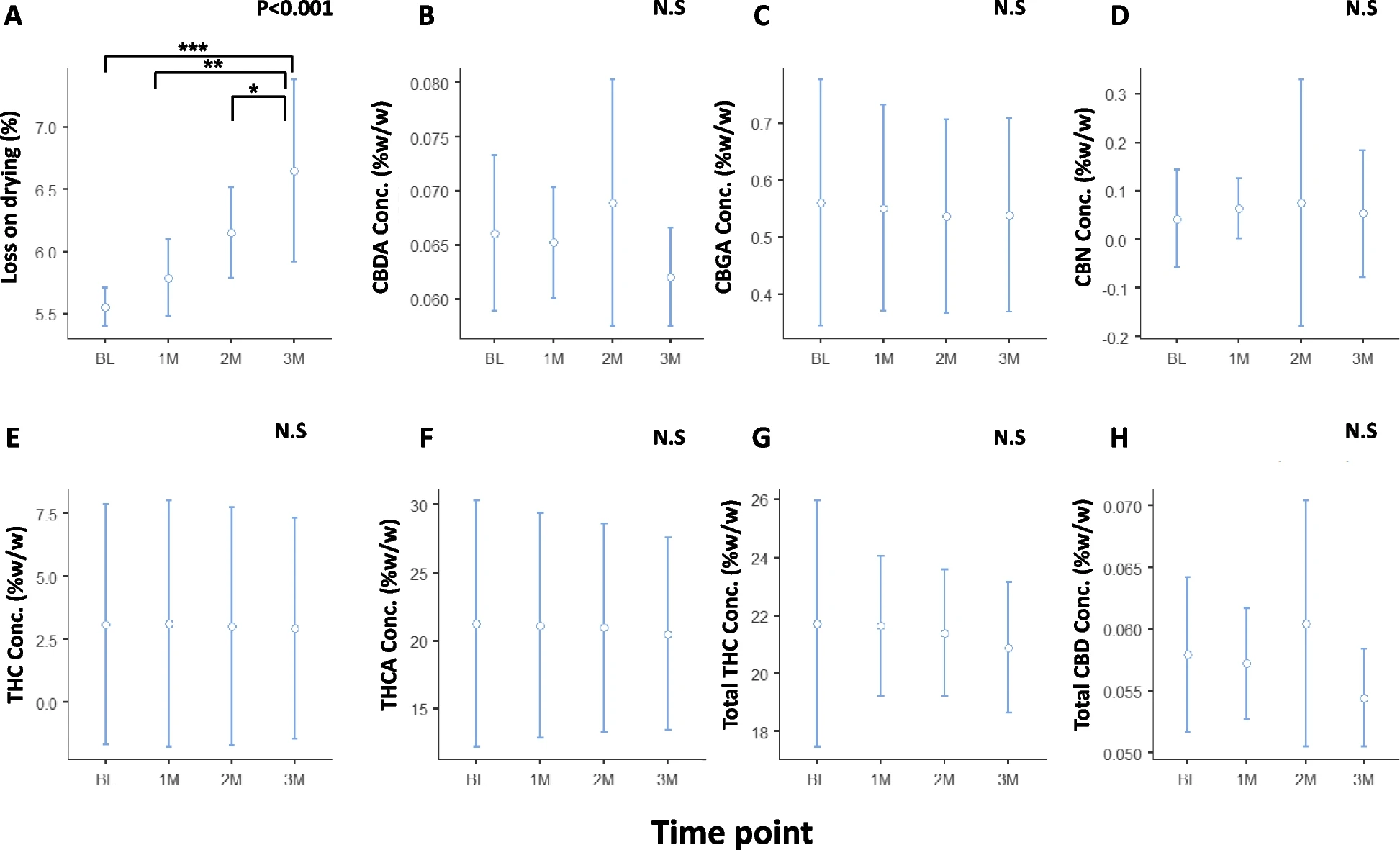
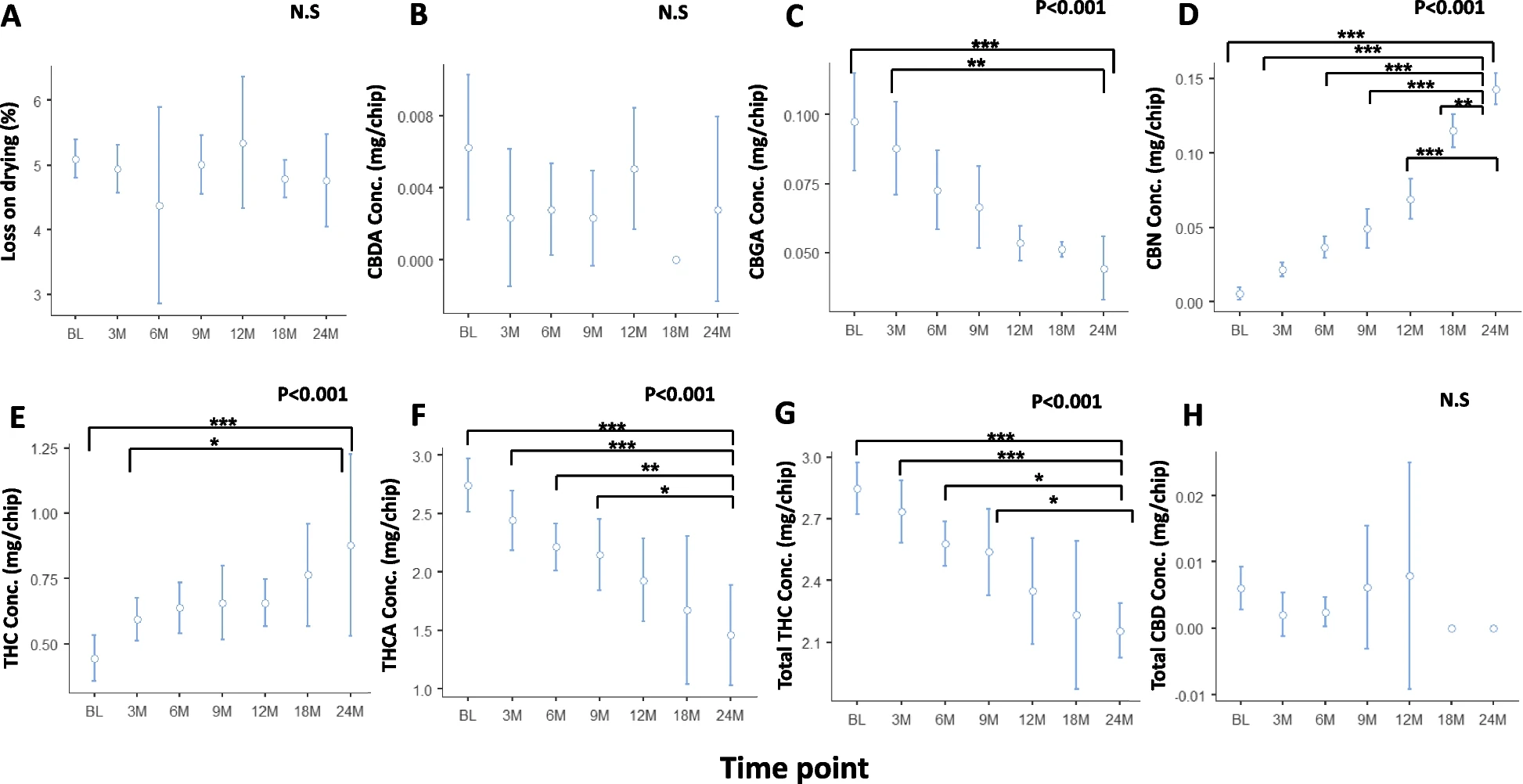
No significant changes were observed in phytocannabinoid concentrations in ground cannabis inflorescence after 3 months of bulk storage in a polypropylene container and sealed in an aluminum foil pouch at 5°C. In contrast, significant changes in phytocannabinoid concentrations were found when ground inflorescence was stored in the Cartridges at 25°C for 2 years. Specifically, CBGA, THCA, and total THC concentrations decreased from 0.097±0.023, 2.7±0.3, and 2.80±0.16 mg/chip at baseline to 0.044±0.007 (55% decrease), 1.50±0.27 (44% decrease), and 2.20±0.083 (21% decrease) mg/chip following 2 years, respectively, while CBN and THC concentrations increased from 0.005±0.005 and 0.44±0.11 mg/chip at baseline to 0.14±0.006 (2700% increase) and 0.88±0.22 (100% increase) mg/chip following 2 years, respectively. Storage at 30°C revealed a steeper change in phytocannabinoid concentrations within an even shorter period. Despite the significant change of relative cannabinoid composition within the Cartridge, the actual THC dose present in the aerosol remained relatively stable throughout this period and within the dosage range of 500mcg±25% required for pharmaceutical-grade Inhalers.
Conclusion
The medical cannabis powder in the SyqeAir Cartridges may be stored at room temperature for at least 2 years after production without affecting the aerosolized THC dose delivered to patients by more than ±25%. Future studies should analyze additional phytocannabinoids and terpenes in the cannabis inflorescence and assess the stability of different cannabis cultivars following storage in the SyqeAir Cartridges.

Evaluating Sex Differences in Efficacy, Safety, and Pharmacokinetics in Patients Treated with Cannabis Using a Metered-Dose Inhaler
Background
Clinical studies thus far examining the effects of medical cannabis treatment on men and women have shown sex-related differences in their responses to the treatment. More specifically, a higher susceptibility to adverse events among women and greater analgesia among men. These studies estimated that these sex differences may be related to a biological or hormonal difference between the sexes or that the finding that women consume completely different cannabis “strains” combinations is the primary cause2. Mentionable, this “strains” difference between the sexes was demonstrated to be related to the consumption of significantly different weight-adjusted doses of many cannabinoids and terpenoids.
Objective
To examine and analyze the differences between women and men and the long-term effectiveness and safety of the treatment using the SyqeAir Metered-Dose Inhaler and Cartridge – a consistent route of administration of medical cannabis that makes use of a single stable “strain” or cultivar (Bedrocan®), which is grown under strict EU-GMP conditions (In-door).
Methods
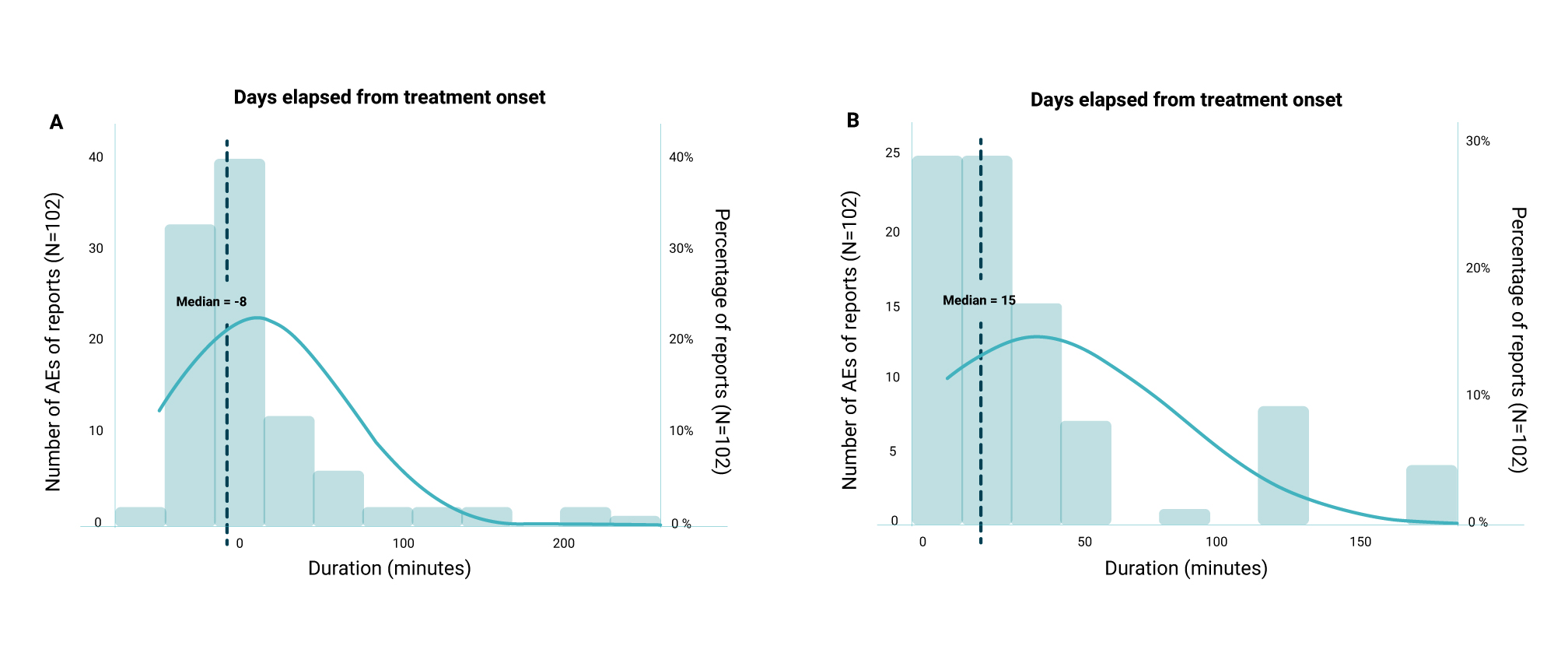
Data collection was performed during the long-term treatment using the SyqeAir Inhaler and Cartridge under real-world conditions. Throughout this process, the patients were closely monitored by the nursing team of Syqe®’s Patient Support Program. ∆9-trans-Tetrahydrocannabinol (∆9-THC) served as a dosage marker for full-spectrum medical cannabis. Up until the data cut-off date (Jan 2023), a total of 1,249 Israeli chronic pain patients were enrolled. 1,120 patients were found eligible for analysis and included in the clinical effectiveness analysis. The mean age of these patients was 60±18 years (54% female), and most (91%) were diagnosed with chronic pain. The patients were assessed for pain intensity using a numeric pain scale (NPS), sleep latency, sleep duration, and sleep quality (based on the PSQI-validated questionnaire) from baseline and over 240 days after treatment initiation. They were also assessed for adverse events (AEs) by reports that were actively collected by Syqe®’s PSP nurses and passively by patients reporting to Syqe®’s Customer Experience Team for over 3.5 years.
Results
No statistical difference was found between men and women in the improvement of the tested measures
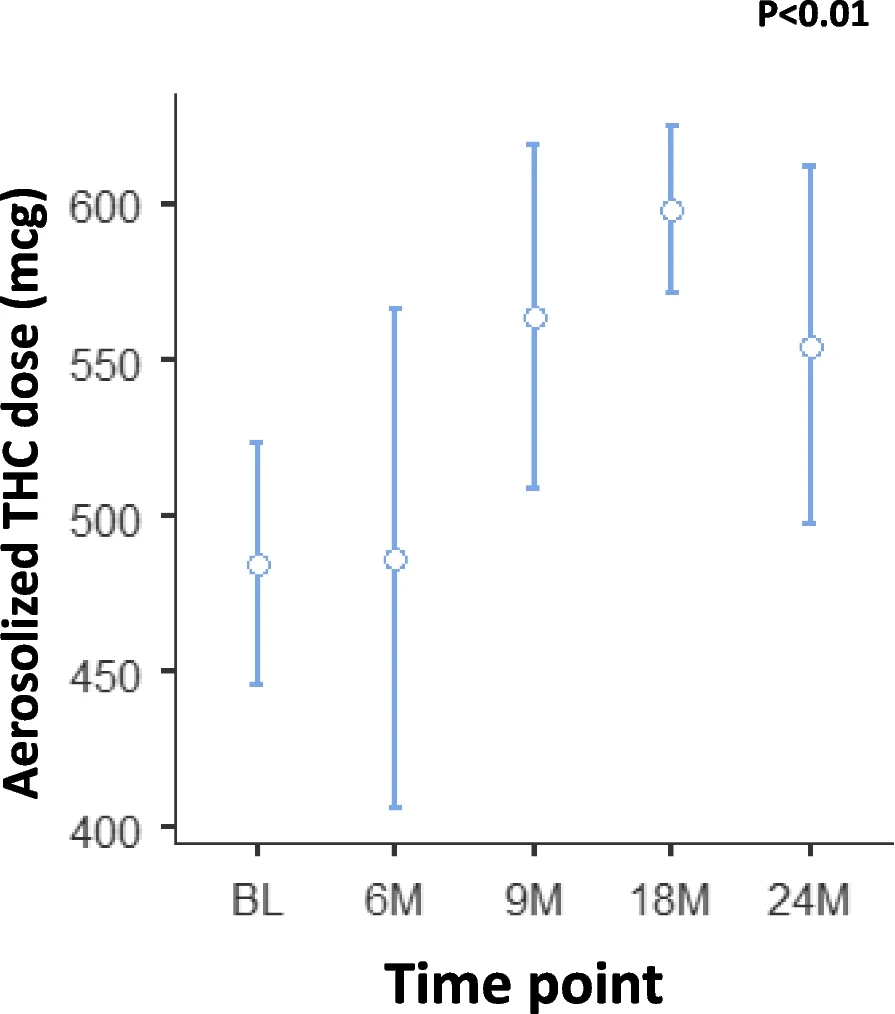
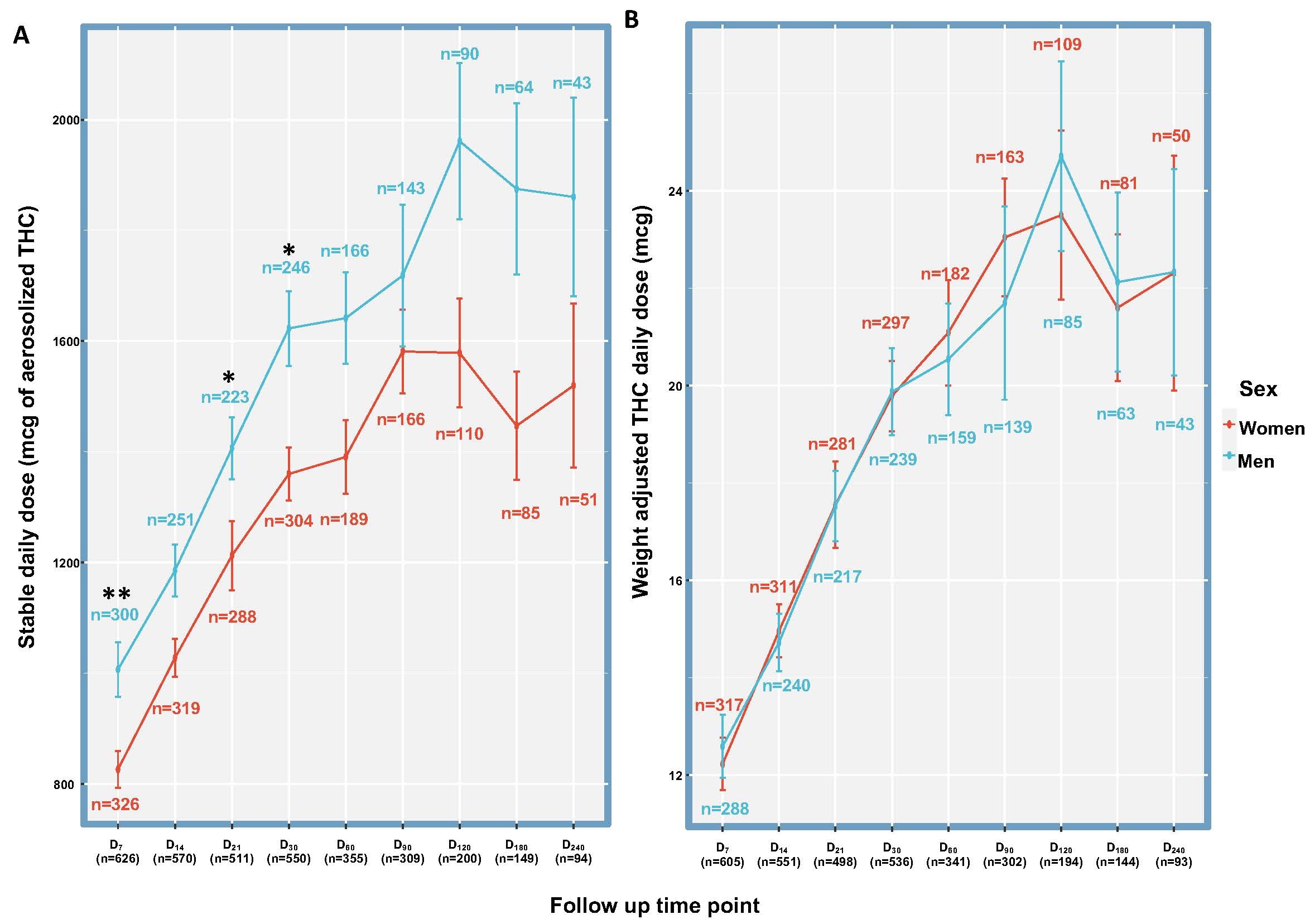
The daily dose stabilized following 90 days of titration at 1,600±1,300 µg aerosolized ∆9-THC. Inhaled ∆9-THC doses did not vary significantly between the sexes (p > 0.05) except in the first month of treatment. Nonetheless, the weight-adjusted dose was almost identical between the sexes (Figure 1).
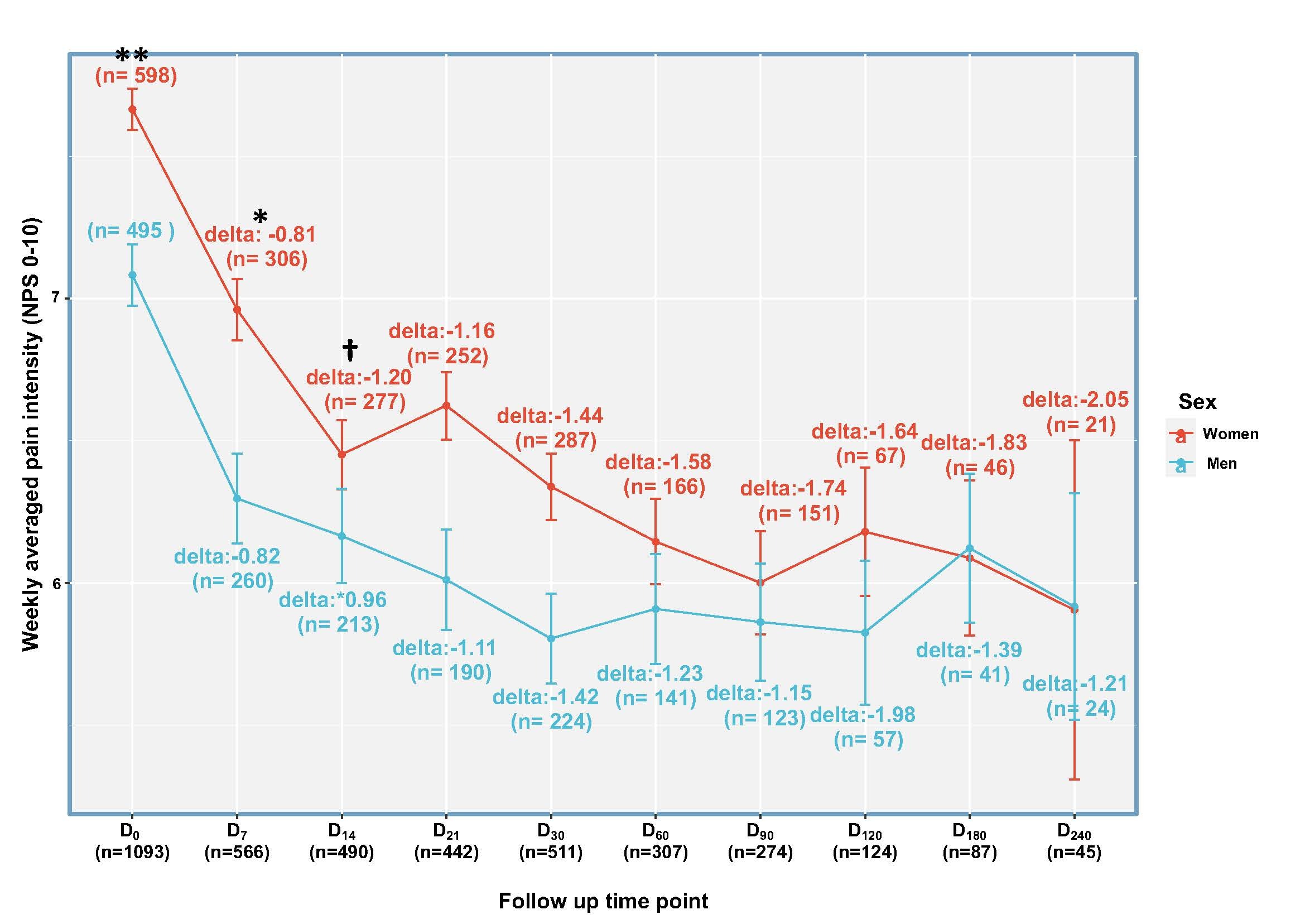
Following the first two weeks, no significant differences were found between the sexes in the effectiveness (Figure 2) or safety (p>0.05) of the medical cannabis treatment. Pain reduction and sleep improvement were similar for both sexes (p>0.05).
An average reduction of 27% in pain intensity
68% of the patients reported a reduction in pain intensity of at least one NPS point, and 26% of the patients reported a≥30% reduction of pain intensity from baseline following the first 30 days of treatment. Following 240 days of treatment with the SyqeAir Metered-Dose Inhaler, an average weekly pain reduction of -1.58 was reported (with a 95% confidence interval ranging from a pain reduction of -0.15 to -3.02). This corresponds with an average of 27% weekly pain intensity reduction. Due to the decrease in the number of participants at the long-term data point of 240 days, a Last Observation Carry Forward imputation was performed that showed a change to an average weekly pain reduction of -2.60.
Improvement in sleep quality: the sleep latency has shortened, and the sleep duration has lengthened
Varying between the sexes and the time points, the sleep latency decreased (improved) between 3 to 31 minutes on average, the sleep duration increased (improved) between 30 to 120 minutes on average, and the sleep quality improved significantly (Figure 3).
Meager rate of adverse events
The overall rate of all-time adverse events reports was equal between men and women and very low at 10% (n = 65, 10% of women and n = 60, 10% of men; χ2 (1) = 0.05, p = 0.820). Only 1.6% of patients reported psychoactive adverse events. The most frequent adverse events were dizziness (n = 45, 4%), cough (n = 32, 3%), headaches (n = 31, 2%), sleepiness (n = 27, 2%), and sore throat (n = 25, 2%). The frequency of all other adverse events reported was <1%.
A secondary analysis of pharmacokinetic data showed no significant differences between the sexes in ∆9-THC and its metabolite pharmacokinetics (Figure 4), in cardiovascular measures, or adverse events severity (p>0.05).
Conclusion
Medical cannabis treatment with the SyqeAir Inhaler and Cartridges demonstrated similar overall long-term pain reduction and a superior adverse events profile compared with the administration of high-dose medical cannabis by conventional routes (smoking, vaporization, oil)3. A significant sleep improvement was also observed in the tested sleep measures.
Additionally, the metered-dose treatment with medical cannabis using the SyqeAir Inhaler, which makes use of a single stable strain (compared to consuming a combination of different strains that has been shown in previous studies to cause differences between the sexes), showed no sex differences in short-term effectiveness, safety, and pharmacokinetics, nor long-term effects, under “real-world” conditions. These findings provide new insights into the treatment with medical cannabis, which may inform clinical practice and policymaking in the field.
Innovation
Metered dosing
In contrast to the previous studies in which the only parameters that were known were the concentration of the ∆9-THC found in the raw plant in a 10gram bag and the monthly consumption (in grams) of the patients, in this current study, it is possible to know what is the dose of ∆9-THC in the aerosol that was emitted to the lungs of each of the patients in each inhalation.
The uniformity of the treatment – the answer to the differences in the effect between men and women
After years in which the conception was that the differences in response between women and men to medical cannabis treatment by inhalation are sex-related or related the different cannabis products – today, for the first time, it can be argued that a metered-dose treatment using the SyqeAir Inhaler and Cartridge can provide similar results for both sexes, with a preferable safety profile, and a pain reduction effects as well as an improvement in sleep quality for many of the treated patients.
- Aviram J, Glezerman M, Hayam E, et al. Evaluating Sex Differences in Efficacy , Safety and Pharmacokinetics in Patients Treated with Cannabis by a Metered-Dose Inhaler. Pharmaceuticals. 2023;16(1426):1-15. doi:doi.org/10.3390/ph16101426
- Aviram J, Lewitus G, Vysotski Y, et al. Sex Differences in Medical Cannabis Related Adverse effects. Pain. 2021;163(5):975-983. doi:10.1097/j.pain.0000000000002463
- Aviram J, Pud D, Gershoni T, et al. Medical Cannabis Treatment for Chronic Pain: Outcomes and Prediction of Response. Eur J Pain. 2020;25(2):359-374. doi:10.1002/ejp.1675









